Mon, Jul 21, 2025
[Archive]
Volume 20, Issue 1 (March 2023)
IJMSE 2023, 20(1): 1-8 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Khoshhal R, Alavi Nezhad Khalil Abad S V. The Effect of Graphite on the Iron-Silicide Compound Formation. IJMSE 2023; 20 (1) :1-8
URL: http://ijmse.iust.ac.ir/article-1-3082-en.html
URL: http://ijmse.iust.ac.ir/article-1-3082-en.html
Abstract: (11104 Views)
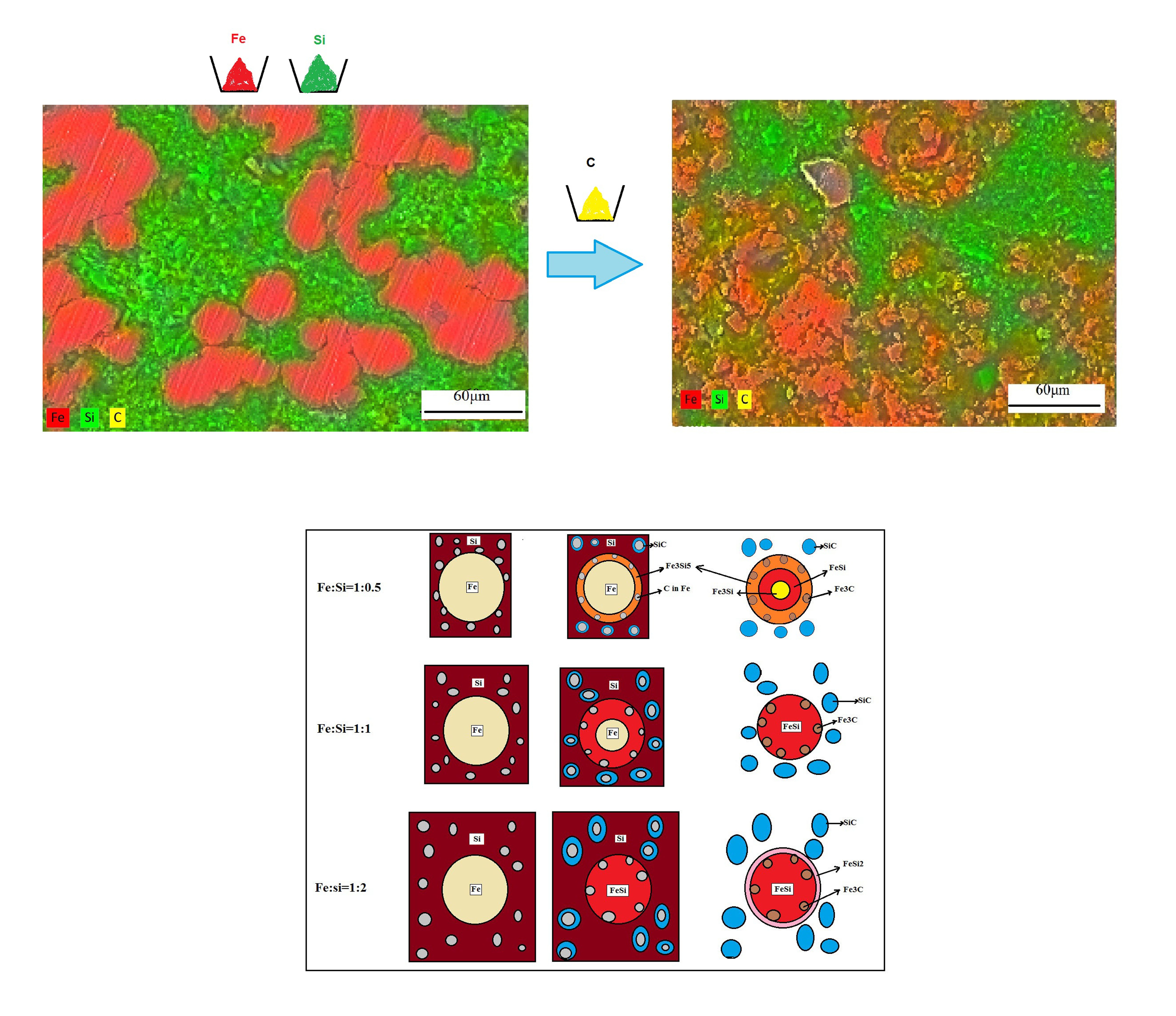
- In this article, the effect of graphite on iron-silicon interactions was investigated. It was found that, as graphite enters the iron structure, it permits further development of iron-silicon reactions. It was found that in the stoichiometric ratio of 1:0.5 of iron and silicon, when graphite is added to the system, simultaneously with the reaction of iron and silicon to form Fe3Si5, some amount of carbon can be dissolved in the iron and lead to more diffusion in iron and more iron silicide production. Silicon also reacts with carbon and produces SiC. The more amount of carbon entered into the system, the more growth of SiC occurs, while the production of other iron silicide phases, namely FeSi and Fe3Si preceded. Finally diffused carbon into the iron reaches a definite amount that can form Fe3C. In the stoichiometric ratio of 1:1 of iron and silicon, the formation of FeSi and SiC phases is observable. At the same time, the diffusion of carbon occurs in the same as the previous stoichiometric ratio. In the stoichiometric ratio of 1:2 of iron and silicon, compared with the stoichiometric ratio of 1:1, a larger amount of silicon is available and, the FeSi2 phase can form in addition to FeSi
Type of Study: Research Paper |
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |